cyclicstudies: Organic Chemistry: Electrophilic Additions to Alkenes Hey all! I wanted to put togeth
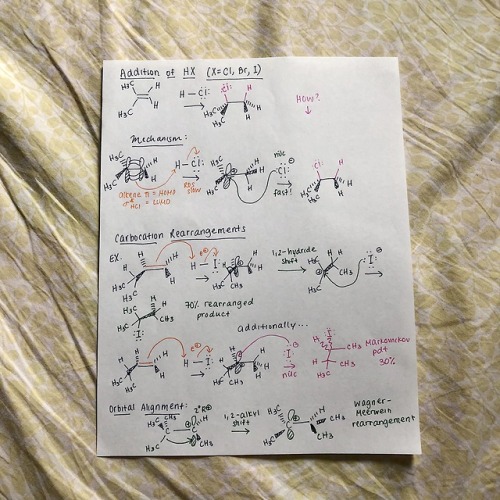
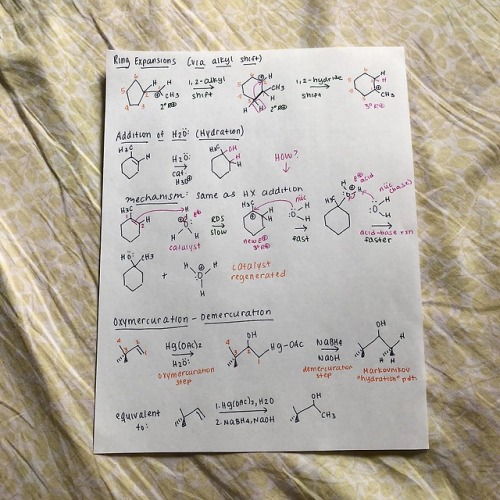
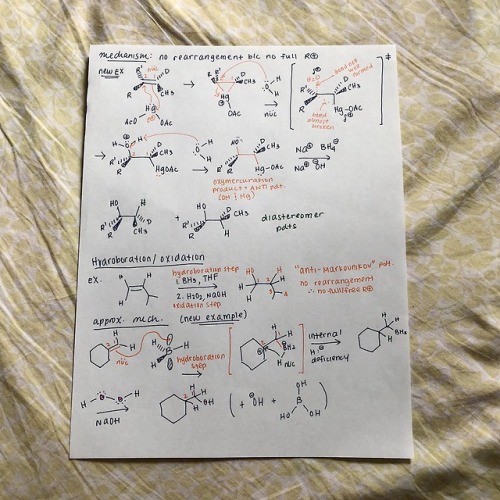
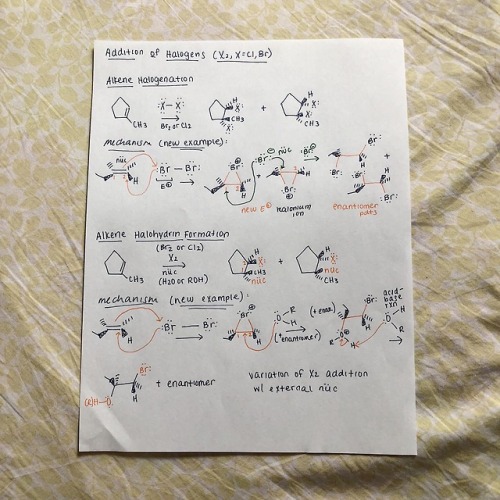
cyclicstudies: Organic Chemistry: Electrophilic Additions to Alkenes Hey all! I wanted to put together a few review guides for the reactions I learned in my organic chemistry 1 class. I’m starting off with the alkene-related reactions, specifically with electrophilic additions. Despite being super hard to fully conceptualize (I still have trouble with them!!), they’re very important to recognize, because the same patterns repeat with other reaction types, particularly with alkynes. I’ll go through each reaction type here with a quick mechanism and jot down important bits of info. But before I start, I used a lot of abbreviations, so here’s a key: R+ is carbocation R is any group with a carbon IM is intermediate X is halogen E+ is electrophile RDS is rate-determining step HOMO is highest occupied molecular orbital LUMO is lowest unoccupied molecular orbital Squiggly lines indicate enantiomers formed due to alternating wedges and dashes Addition of HX Introducing Markovnikov’s rule: the formation of a more stable R+ is favored b/c it’s a lower energy process Alkene additions proceed faster via a more stable R+ IM (kinetics)Tri-substituted carbons are favored over mono-substituted carbons X is added to the more substituted carbon Always watch for stereochemistry! Enantiomers can be formed as products Carbocation Rearrangements Carbocation rearrangements will occur so the R+ is as stable as possible That’s why the Markovnikov product won’t always be the major product Rearrangements usually occur through hydride shifts, but alkyl shifts can also happen if that’s the only possible route Remember: always check to see where the positive charge is at− if you can shift a hydrogen or methyl group over to make that carbon extra substituted, then it’s probably right With the orbital alignment (aka alkyl shift), there’s a filled-empty orbital overlap, where the migrating bond is aligned with the empty 2p orbital Ring Expansions Some questions are gonna ask you to do ring expansions, so always think alkyl shifts (again, this is so we form the most stable R+) NUMBER. YOUR. CARBONS. This will get messy Note that even though the di-substituted carbon doesn’t change after the first arrow, the shape changes from cyclopentane to cyclohexane, so it’s thermodynamically more stable Hydration This follows Markovnikov’s rule− the OH group is always on the more substituted carbon of the alkene Also this is pretty much a repeat of HX addition, but with some acid-base action Pro-tip: your catalyst, H3O+, is always gonna be the electrophile, and your nucleophile is always gonna be the double bond, so START THERE! There’s gotta be water after that first step (hydration, duh) Your water is now a nucleophile, and it’s gonna start attacking the R+ Remember to follow through with any possible R+ rearrangements− we won’t need it here b/c the R+ is already tri-substitutedYour catalyst MUST be regenerated!! *insert acid-base reaction* Side note: alkene dehydration is the reverse of hydration and is an E1 process DON’T ADD H2O/H3O+ UNDER DEHYDRATION CONDITIONS Instead, use a strong acid, like concentrated H2SO4 Oxymercuration-Demercuration This is essentially an alkene “hydration” w/o the rearrangement part We don’t have rearrangement b/c the mercurinium ion doesn’t have a carbon When the mercurinium ion bridge breaks, the nucleophile (in the second example, water), attacks the MORE substituted carbon, whereas the HgOAc attacks onto the LESS substituted carbon The OH and HgOAc add ANTI to each other, but then the H from BH4- changes orientation on the LESS substituted carbon (forming diastereomers) Side note: as a variation, you can also use an alcohol (ROH) instead of H2O in the oxymercuration step Hydroboration/ Oxidation The double bond nucleophile attacks the empty orbital in BH3 The BH3 attaches onto the LESS substituted carbon, so the positive charge builds on the MORE substituted carbon I like drawing the third H in BH3 as an extension of BH2, so I can visually see the hydride shift The OH from the peroxide basically replaces the BH2 The specific steps with BH3 get messy, so I was taught a revised method− basically, a concerted addition of BH3 w/ an internal H- transferThere is regioselectivity in the sense that the partial positive charge follows Markovnikov’s rule There is stereospecificity due to the syn (same side) addition of H and BH2 in the hydroboration product Alkene Halogenation Note that I2 doesn’t work here as a possible halogen Markovnikov regioselectivity is present There is ANTI stereospecificity, as in the two individual X’s from X2 attach themselves as opposite wedges and dashes Alkene Halohydrin Formation This is very similar to the previous alkene halogenation, except the second X in X2 doesn’t attach onto the final product Rather, the nucleophile replaces the second XMarkovnikov regioselectivity is also present (this is SN1-like) There is ANTI stereospecificity yet again (this is SN2-like) And that’s it! Well, this is a good chunk of alkene reactions (and a pretty brief version of it). Regardless, I hope this is helpful! I hope to follow up with extra review mechanisms for additional alkene and alkyne reactions. Thanks for reading!! -- source link